

The Challenge
The volume of regulatory information requests continues to grow.
Large chemical and consumer goods companies can receive upwards of 40,000 compliance related requests a year from customers, partners, authorities and third-party certification organizations.
Finding the relevant data and documents to respond to these requests is a time-consuming process that often requires traversing multiple systems of record. The result is delays, inefficiencies and increased risk of major compliance communication errors.
The Solution
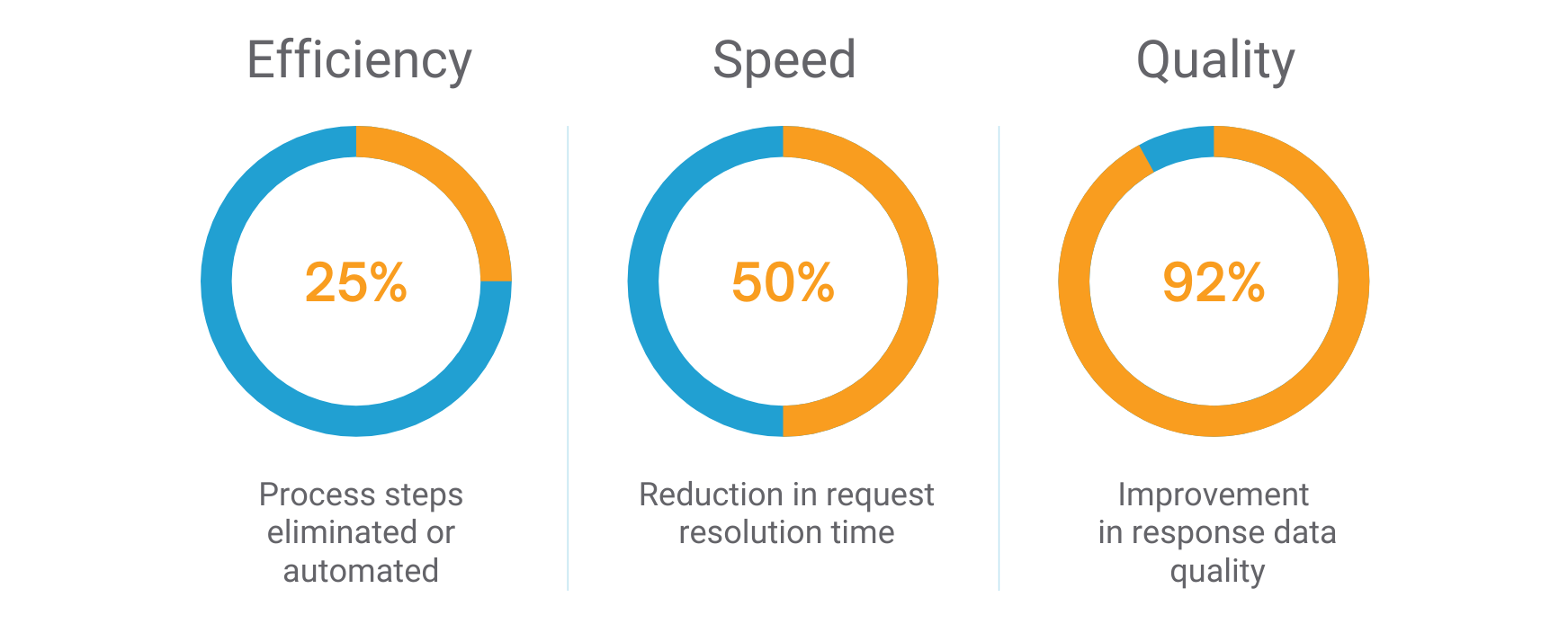
Ensure speed, efficiency, consistency and accuracy to your responses with Veeva RegulatoryOne.

End-to-end Request Management
Request and response data, documents and workflows are unified in a transparent, single system of record.

Transformational Efficiency
Metadata-driven process automation dramatically streamlines work and maximizes efficiency.

Consistency and Accuracy
The system ensures only approved content is shared. The "similar requests" feature maximizes global consistency and response reusability.

Real-time KPIs
Built in reports and dashboards provide real-time insight into customer needs, response lead times, bottlenecks and trends.
Benefits
Demo
Respond to customers and other stakeholders faster and more accurately using Regulatory Request Management capabilities.
Customer Success

"Global collaboration is crucial for us. It is essential to have one source of regulatory information instead of distributed, siloed systems. High availability, reliability, and performance are key. With Veeva Vault, we have a good system in place that supports our needs."
Armin Sauer, Team Lead Global Regulatory IT Services at BASF
Improve your agility, transparency, and compliance with Veeva's Cloud solutions for
regulatory, quality, studies and advertising claims management.

