Unified Quality, Training and H&S Management
Shift your organization towards predictive quality and safety by unifying processes under a single cloud-based platform.
Learn more
CHALLENGE
In a business environment of ever-increasing standards and stricter oversight, CPG organizations need a unified solution that can connect quality, H&S, and employee training at all levels of the organization. Maintaining functional silos that utilize outdated tools has created cost inefficiencies, increased risks, and a lack of transparency which together are a barrier to creating a strong culture of safety and quality. To stay competitive, these organizations need to dissolve these barriers and maintain a state of always-on operational excellence.

VEEVA’S APPROACH
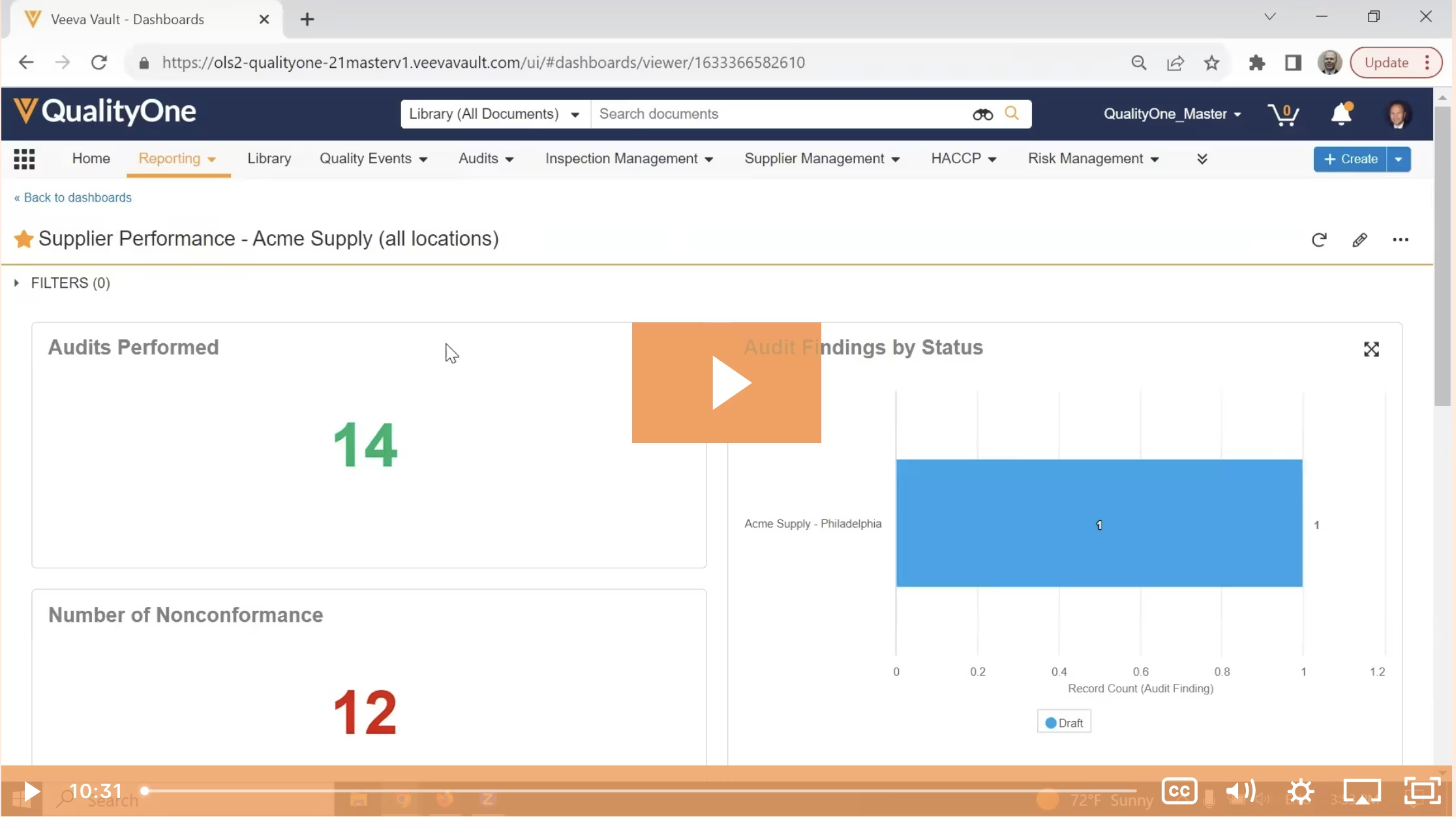
Veeva Vault is a proven platform for managing Quality, H&S, and Training at enterprise scale. With seamless mobile and desktop capabilities, powerful out-of-the-box capabilities, and a flexible, highly configurable platform that can adapt quickly to your business needs, you can increase efficiency, transparency, and engagement across your enterprise.
GxP Document and Data Management
Capture, standardize, and centralize critical product stewardship and quality management content on a single platform.
Learn more
CHALLENGE
Regulatory and Quality teams can spend nearly 50% of their time managing information. Much of that time is spent on non-value-added activities such as finding documents, manually reentering data, or verifying information with suppliers, customers, and the business. This is because required information is scattered across spreadsheets or locked in complex transactional systems that are fragmented, inconsistent, and hard to access.
Learn about our Quality Document Management Capabilities »
Learn more about our Regulatory Suite of Document and Data Management »

VEEVA’S APPROACH
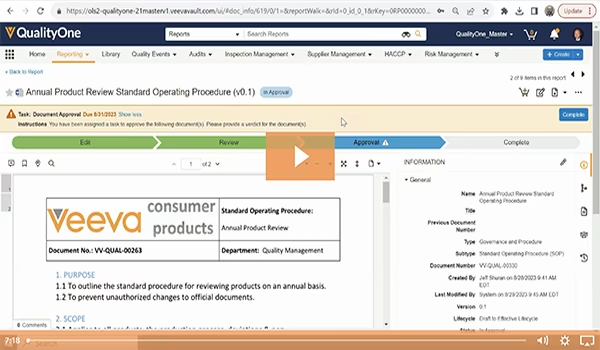
Veeva Consumer Products' specific applications are built on a proven platform for managing GxP content in the cloud. The solution eliminates disconnected spreadsheets and integrates with your ERP and PLM system to liberate data and make it accessible to regulatory and quality teams in real-time. The result is greater control and standardization of your mission-critical controlled content processes. This leads to increased productivity as people can access, reuse and report on information more rapidly. Standardizing controlled content on a single platform also creates cost savings through process simplification, task automation, and the generation of insight from data.
Product and Raw Material Compliance Management
Accelerate time to market by flexibly adapting to changing customer and regulatory requirements.
Learn more
CHALLENGE
Demand for trusted and transparent products has increased drastically. Consumers want to know how a product might affect their health, the quality of its production processes, and the sustainability of its ingredients. Government and non-government organizations are responding in kind with new regulations, standards, and labels. Reducing time-to-market while meeting these evolving consumer preferences and shifting compliance requirements puts tremendous pressure on CPG Product Compliance teams.

VEEVA’S APPROACH
Veeva Consumer Products solutions for regulatory management ensure that your products are always in complete compliance with evolving customer and regulatory requirements. These applications enable you to simplify and digitalize core regulatory processes like responding to compliance information requests, performing raw material assessments, sending supplier questionnaires, and supporting the development of compliant product claims. The result is a more strategic compliance function that improves operational efficiency, grows brand trust, and provides the business with the ability to accelerate new product introduction in a way that wins the hearts of the new socially conscious consumer.
Supply Chain Collaboration
Significantly reduce supplier risk while building a compliant, cost-effective, and continuously improving the supply chain.
Learn more
CHALLENGE
Modern quality management and product stewardship no longer stops with the four walls of your company. The CPG value chain is now so connected that the quality failure of a supplier can be as costly as the failure of your own quality system. But traditional methods of assessing and qualifying raw materials and collaborating with suppliers on quality management processes are burdensome and time-consuming, and distract from your own team's quality and product compliance objectives.
Learn more about how our regulatory suite can help collecting essential supplier information »
Learn about the supplier management capabilities within our Quality Suite »

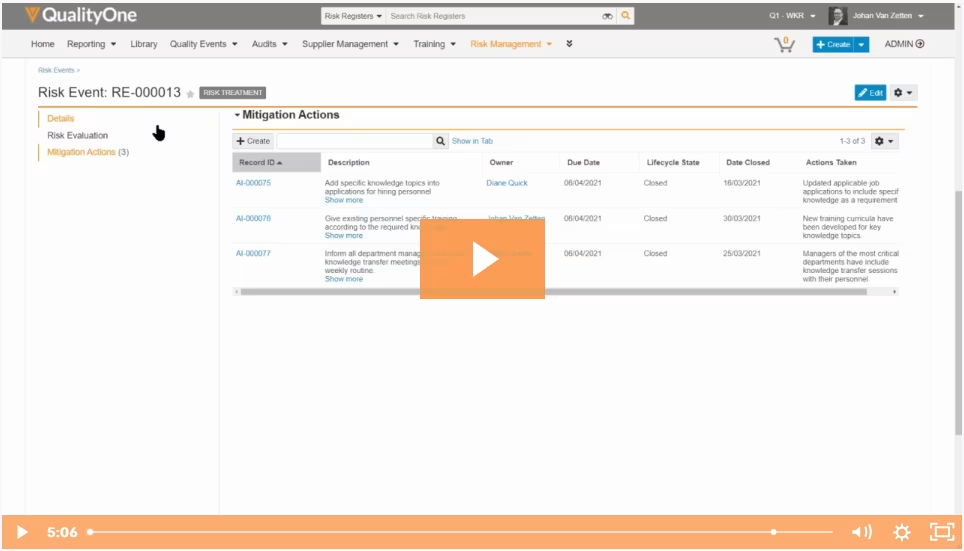
VEEVA’S APPROACH
Veeva Consumer Products solutions are built on a proven platform that embeds control and transparency into your supplier relationships, and simplifies partner collaboration. The application structures, strengthens, and automates key processes from vendor qualification and audits to sending supplier raw material questionnaires and processing incoming certificates of analyses. User-level permissions and built-in data integrity controls enable you to provide in-system access to suppliers so you can directly integrate them into your quality processes. The result is increased operational agility from more collaborative supplier relationships and increased efficiency through automated data transfer that eliminates the need for endless scanning of documents, copy and pasting, and transcribing data.
Product Claims Management
Increase the speed of innovation and reduce risk with effective and efficient digital management of product and advertising claims across the enterprise.
Learn more
CHALLENGE
Advertising and product claims are critically important to CPG companies, helping to define functional product benefits, differentiate from the competition, support brands, and, most importantly, build trust with consumers. If consumer trust is at the heart of strong brands, then advertising and product claims are at the heart of building that trust. However, the process of developing claims, substantiating, adapting to local markets, and monitoring usage is complex, involves multiple stakeholders, and can involve significant regulatory and legal risk for companies. Many companies today use disparate, manual systems such as spreadsheets, file share folders, and email to manage their claims process. There’s often no single source of truth for approved claims, and comments and approvals get lost in long email chains. This not only slows innovation and speed to market for claims and the products and campaigns that depend on those claims, but it also presents a very real risk of damaging the consumer trust that brands have worked so hard to build.

VEEVA’S APPROACH
Veeva Claims is a cloud-based application designed specifically to manage the end-to-end lifecycle for advertising and product claims, as well as the cross-functional collaboration required for this critical process that involves R&D, legal, regulatory, marketing, and other functional groups. With core claims management, as well as capabilities for substantiation, localization of global claims, challenge-response, packaging copy management, and oversight and insights into the global claims process, Veeva Claims helps companies accelerate time to market, reduce risk, protect their brands, and ultimately build and maintain consumer trust. Veeva Claims is the single source of truth, transparent, scalable, and global.
ESG & Sustainability
Build confidence in today’s dynamic and uncertain regulatory world of ESG and sustainability.
Learn more
CHALLENGE
Consumers increasingly demand that companies be vocal and transparent about what they do to address ESG and sustainability concerns and how their products are being developed and sourced. While regulations are spotty, the rate at which they are being implemented globally is increasing. CPG companies are under particular scrutiny as consumers use these products daily and are very aware of the ingredients. Competition can be fierce, and the need to make claims about a product or corporate sustainability is acute. Frequent purchases and disposal make the long-term impact of packaging a constant source of concern. In addition, ESG and sustainability data and documentation up and down the value chain are often difficult to obtain, track and manage due to the many disparate and siloed systems used today. Companies are in the difficult position of having to be transparent about their products and corporate targets but need the tools to feel confident in what they are saying.

VEEVA’S APPROACH
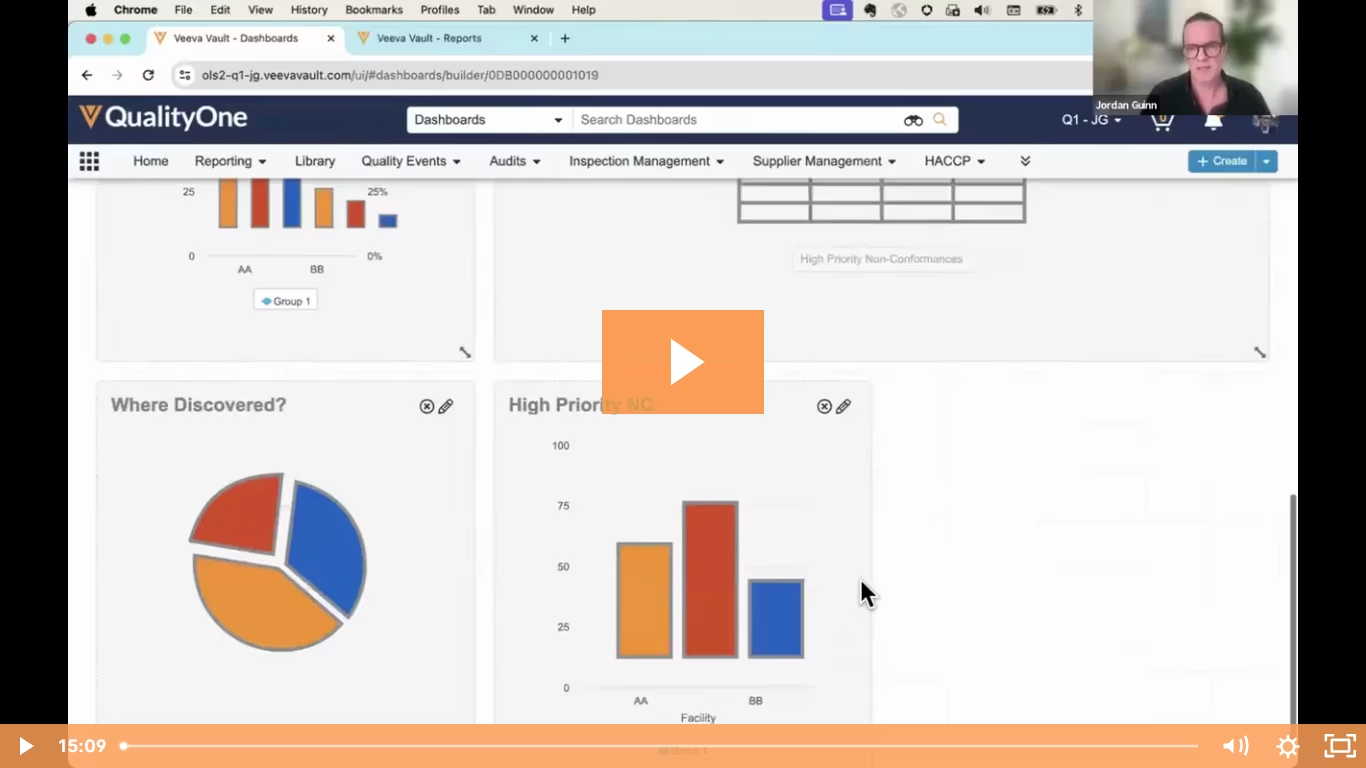
Veeva can help bring order to many critical areas related to ESG and sustainability. With several applications to serve the product journey, all built on a common platform, Veeva can bring ESG and sustainability-related data and documents together to suit the needs of the many diverse teams working on sustainability priorities within companies. From requesting and managing data or documentation from suppliers related to sustainable formulations and raw materials, to managing dossiers and usage of third party certifications across brands globally, or managing the complex world of ESG and sustainability claims at the product or corporate level - Veeva is a partner in helping CPG companies bring order to the often chaotic yet critically important area of ESG and sustainability.

CHALLENGE
The task of managing food safety and quality is becoming more complicated. Between supply chain disruptions, sustainability requirements, building and maintaining a food safety culture, and pressures on manufacturing to keep the global food supply chain running, F&B organizations need ever-increasing agility. New and changing regulatory requirements make it difficult to stay current and compliant. Global supply chains mean more outsourcing and more requirements. An expanding product portfolio puts further strain on limited quality resources. Companies lack a unified view of all the data in the context of business operations and a structured process for communicating and sharing data with suppliers and external partners. The result is often an inability to extract information and insights from siloed data, so that they can stay ahead of, and adapt to, emerging risks because quality and food safety teams end up too focussed on maintaining processes instead of applying their expertise to interpreting the outcome of processes.







-logo@2x.png?width=188&height=98&name=Veeva-Reckitt_(2021)-logo@2x.png)