Unified Management Systems for Quality, H&S and Training
Drive operational excellence and improve QHSE performance with a modern, agile and digital approach.
Learn more
CHALLENGE
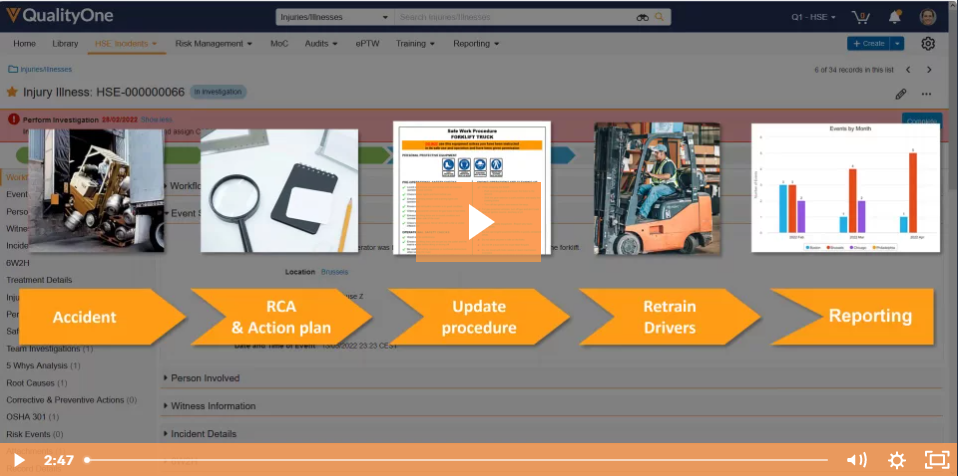
Quality, H&S and Training processes are typically managed within a location with functional silos using outdated tools, leading to cost inefficiencies, increased risks, lack of transparency, and headwinds to creating a strong culture of safety and quality.

Veeva’s Approach
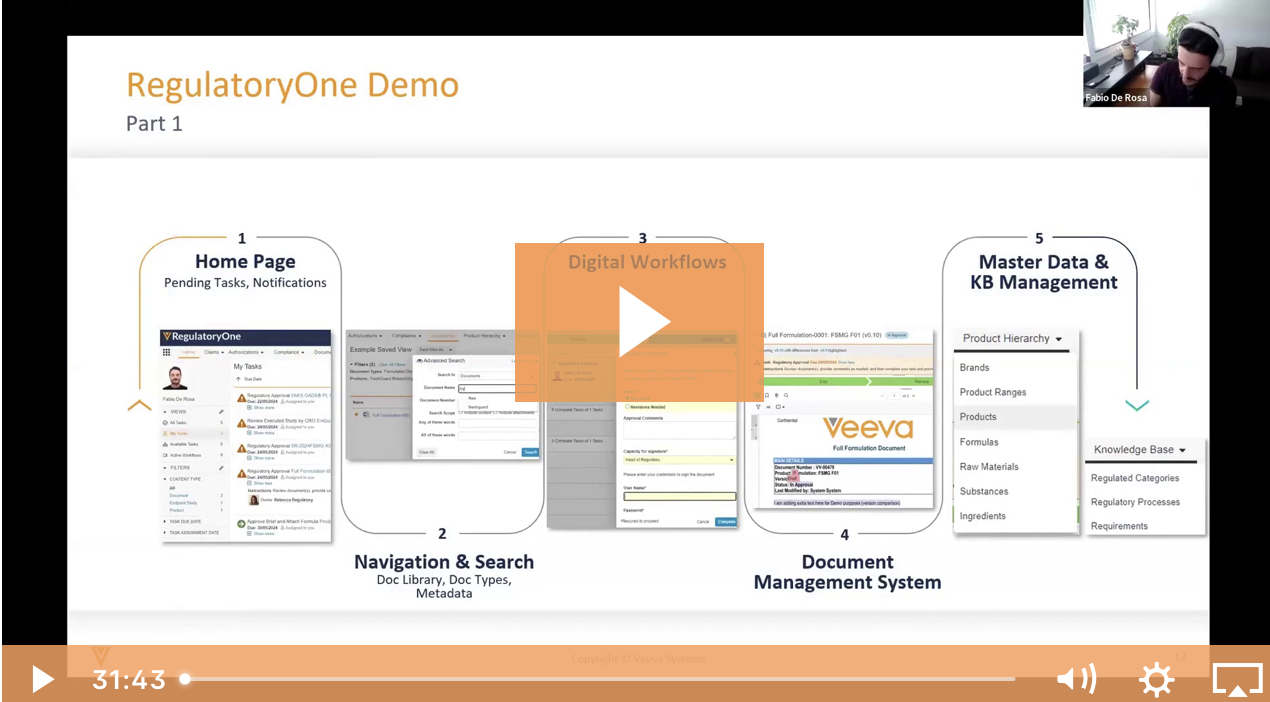
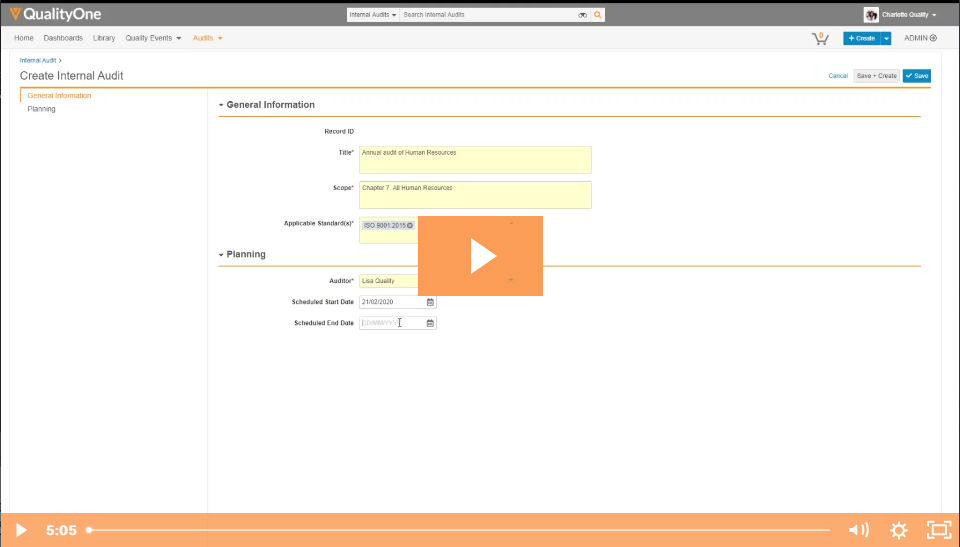
Veeva Vault is a proven platform for managing Specialty Chemicals' Quality, H&S, and Training at enterprise scale. With seamless mobile and desktop capabilities, powerful out-of-the-box capabilities, and a flexible, highly configurable platform that can adapt quickly to your business needs, you can increase efficiency, transparency, and engagement across your enterprise.
A Cloud Platform that Enables Innovation at the Pace of Customers and Consumers
Become supplier-of-choice with modern software solutions to meet the agility, speed and differentiation of the beauty, personal, and home care industries.
Learn more
CHALLENGE
The beauty, personal, and home care industry is characterized by unrelenting demands for product innovation and speed to meet rapidly evolving consumer needs and hypersegmentation. Delivering products to customers that meet a complex array of consumer, customer, government, NGO and sustainability requirements requires new tools that unlock product data, break organizational silos and enhance collaboration.

Veeva’s Approach
Veeva Vault is the platform of choice for the world's most prestigious Beauty, Personal, and Home Care Companies and their Specialty Chemical partners. By connecting people, processes, and data inside the organization and along the supply chain, product transparency and sustainable innovation are accelerated and differentiating, margin-accretive product benefits and claims are unlocked.
Supply Chain Collaboration
Eliminate supplier risk while building a compliant, cost-effective and continuously improving supply chain.
Learn more
CHALLENGE
Modern quality management and product stewardship no longer stops with the four walls of your company. The value chain is now so connected that the quality failure of a supplier can be as costly as the failure of your own quality system. But traditional methods of assessing and qualifying raw materials and collaborating with suppliers on quality management processes are burdensome and time-consuming, and distract from your own team's quality and product compliance objectives.

Veeva’s Approach
Veeva's digital solutions embed control and transparency into your supplier relationships and simplifies partner collaboration. The application structures, strengthens, and automates key processes from vendor qualification and audits to sending supplier raw material questionnaires and processing incoming certificates of analyses. User-level permissions and built-in data integrity controls enable you to provide in-system access to suppliers so you can directly integrate them into your quality processes. The result is increased operational agility from more collaborative supplier relationships and increased efficiency through automated data transfer that eliminates the need for endless scanning of documents, copy and pasting, and transcribing data.
Cloud Solutions for Enterprise GxP Compliance
Meet the compliance demands of crop protection, tobacco, consumer health and pharma customers.
Learn more
CHALLENGE
As a supplier of APIs, Biocides, Pesticides or other highly regulated chemicals, compliance cannot be compromised. Audit readiness anytime, anywhere, is a crucial element of a fully compliant and highly efficient management system.

Veeva’s Approach
Veeva Vault is the platform of choice for both the world’s leading pharma and crop protection companies and their suppliers. Built from the ground-up with validation, product compliance and speed as the focus, our solutions for Quality, LIMS, Training and more will provide your organization with the confidence and capability to be the trusted supplier of choice.
Learn more

Lorem Ipsum
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim.

CHALLENGE
The task of managing food safety and quality is becoming more complicated. Between supply chain disruptions, sustainability requirements, building and maintaining a food safety culture, and pressures on manufacturing to keep the global food supply chain running, F&B organizations need ever-increasing agility. New and changing regulatory requirements make it difficult to stay current and compliant. Global supply chains mean more outsourcing and more requirements. An expanding product portfolio puts further strain on limited quality resources. Companies lack a unified view of all the data in the context of business operations and a structured process for communicating and sharing data with suppliers and external partners. The result is often an inability to extract information and insights from siloed data, so that they can stay ahead of, and adapt to, emerging risks because quality and food safety teams end up too focussed on maintaining processes instead of applying their expertise to interpreting the outcome of processes.